The electrospinning is a widely implemented technique for the fabrication of nanofibers. This technique relies on a DC applied potential between a syringe tip and a substrate, typically an aluminum foil. The versatility of the electrospinning has found numerous applications such as tissue engineering, biosensors, filtration, wound dressing, drug delivery, and enzyme immobilization. The classical setup is depicted in Figure 1 (Davis et al., 2015) The diameter of the fibers fabricated by this technique range from 2nm to a few micrometers. The electrospinning technique allows obtaining materials with smaller pores and higher surface areas compared to standard mechanical fiber-spinning technologies. Other advantages of this nanofiber production technique are a high surface-to-volume ratio, tunable porosity, malleability (architecture control), and the control of the nanofiber composition (Bhardwaj and Kundu, 2010).
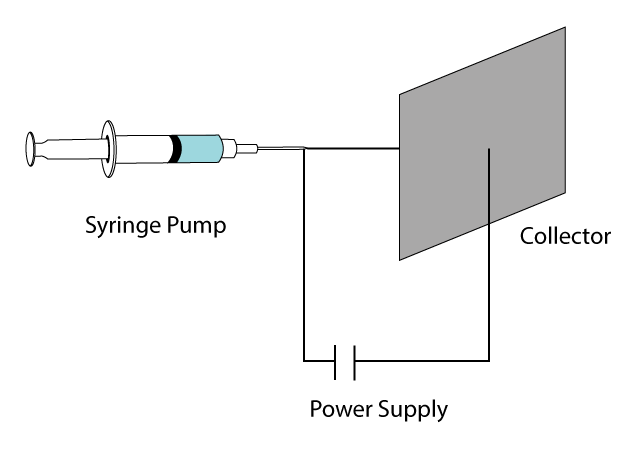
Fig. 1. Schematic representation of a classical electrospinning setup
Electrospinning for tissue engineering.
Electrospun nanofibers are an exciting tool for architecture control in engineered cardiac tissue. For the nanofibers deposition synthetic biodegradable polymers have been used like polyurethane, polycaprolactone(PCL), polylactic acid (PLA), polyglycolic acid (PGA), and more recently polymethylglutarimide (PMGI) at very slow flow rates from 2-5 mL/h. The syringe pumps from Chemyx as Fusion 100 are suitable for the electrospinning process (Orlova et al., 2011). A fine tune control allows the alignment of nanofibers, which greatly influence cell shape and the general architecture of tissue monolayers.
The Polyethylene oxide (PEO) is another polymer that has great attention for tissue engineering due to is a nontoxic biocompatible polymer. The development of scaffolds for tissue engineering and the matrix system for controlled drug release are the main applications of this polymer. Tensile properties as the Young’s modulus of crosslinked PEO mats was increased by 377.5 and 190.5% compared to those of uncrosslinked PEO mats. The author showed that PEO targets for rapid response systems in tissue engineering (Zhou et al., 2012).
More recently, polylactic acid nanofibers have emerged as an alternative for the reinforcement of epoxy composite systems. The polylactic reinforced fibers obtained with a Chemyx Fusion 100 pump produced a flexural modulus and strength increase of about 50.8 and 31.6% at a 5 and 10 wt % fiber content, respectively. Biopolymers for packaging another daily life application may be favored with these methodologies (Dong et al., 2014).
Development of smart antimicrobial materials through electrospinning.
Beyond the medical applications, carbon nanofibers (CNFs) are highly valuated for microbial control; the CNFs conjugated with silver show activity against Gram-positive Staphylococcus aureus and Gram-negative Escherichia coli. The CNFs were obtained as in the previous examples with a syringe pump and an applied voltage. The strategy allowed the obtainment of a material with outstanding antimicrobial performance (Song et al., 2015). On the other hand, applications as metal sensing are possible. Other interesting nanofiber materials for microbial control are the zein fibers with eucalyptus essential oil which showed antimicrobial activity against Gram-positive and gram-negative bacteria. The electrospun process allowed ultrafine fibers with continuous and homogeneous microstructure.
Conclusion
The development of smart nanofiber materials for biomedical, antimicrobial and other applications can be achieved through Chemyx syringe pumps implementation, which allows a fine tune control of the flow rate. The nanofiber distribution is crucial for the above-mentioned applications and is closely related to the flow rate. To achieve a constant low flow rate a reliable syringe pump is required. If the parameters are optimized properly for high-quality micro, and nanofibers are obtained with outstanding performance.
References:
- Bhardwaj, N., Kundu, S.C., 2010. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 28, 325–347. https://doi.org/10.1016/j.biotechadv.2010.01.004
- Davis, F.J., Mohan, S.D., Ibraheem, M.A., 2015. Electrospinning: Principles, Practice and Possibilities. Electrospinning Princ. Pract. Possibilities 1–18. https://doi.org/10.1039/9781849735575
- Dong, Y., Mosaval, T., Haroosh, H.J., Umer, R., Takagi, H., Lau, K.T., 2014. The potential use of electrospun polylactic acid nanofibers as alternative reinforcements in an epoxy composite system. J. Polym. Sci. Part B Polym. Phys. 52, 618–623. https://doi.org/10.1002/polb.23467
- Orlova, Y., Magome, N., Liu, L., Chen, Y., Agladze, K., 2011. Electrospun nanofibers as a tool for architecture control in engineered cardiac tissue. Biomaterials 32, 5615–5624. https://doi.org/10.1016/j.biomaterials.2011.04.042
- Song, K., Wu, Q., Zhang, Z., Ren, S., Lei, T., Negulescu, I.I., Zhang, Q., 2015. Porous Carbon Nanofibers from Electrospun Biomass Tar/Polyacrylonitrile/Silver Hybrids as Antimicrobial Materials. ACS Appl. Mater. Interfaces 7, 15108–15116. https://doi.org/10.1021/acsami.5b04479
- Zhou, C., Wang, Q., Wu, Q., 2012. UV-initiated crosslinking of electrospun poly(ethylene oxide) nanofibers with pentaerythritol triacrylate: Effect of irradiation time and incorporated cellulose nanocrystals. Carbohydr. Polym. 87, 1779–1786. https://doi.org/10.1016/j.carbpol.2011.09.095
